4-Dimethylaminopyridine: A Versatile Catalyst in Organic Synthesis
Related Articles: 4-Dimethylaminopyridine: A Versatile Catalyst in Organic Synthesis
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to 4-Dimethylaminopyridine: A Versatile Catalyst in Organic Synthesis. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: 4-Dimethylaminopyridine: A Versatile Catalyst in Organic Synthesis
- 2 Introduction
- 3 4-Dimethylaminopyridine: A Versatile Catalyst in Organic Synthesis
- 3.1 Unveiling the Structure and Properties of DMAP
- 3.2 Deciphering the Mechanism of DMAP Catalysis
- 3.3 Applications of DMAP: A Catalyst for Diverse Transformations
- 3.4 Advantages of DMAP: A Catalyst with Multiple Benefits
- 3.5 FAQs about DMAP: Addressing Common Questions
- 3.6 Tips for Using DMAP as a Catalyst: Practical Guidance
- 3.7 Conclusion: The Enduring Importance of DMAP in Organic Synthesis
- 4 Closure
4-Dimethylaminopyridine: A Versatile Catalyst in Organic Synthesis

4-Dimethylaminopyridine (DMAP) is a potent organic catalyst that has revolutionized various synthetic strategies in organic chemistry. Its remarkable ability to accelerate reactions, particularly those involving acyl transfer, has made it an indispensable tool for researchers and industrial chemists alike. This article delves into the multifaceted nature of DMAP, exploring its structure, mechanism of action, applications, and advantages, highlighting its crucial role in modern organic synthesis.
Unveiling the Structure and Properties of DMAP
DMAP is a heterocyclic compound characterized by a pyridine ring with a dimethylamino group at the 4-position. This specific arrangement imparts crucial properties that underpin its catalytic prowess. The electron-donating nature of the dimethylamino group significantly increases the electron density on the nitrogen atom of the pyridine ring, making it a potent nucleophile. This nucleophilicity is further amplified by the resonance effect, which delocalizes the lone pair of electrons on the nitrogen atom across the aromatic ring.
This unique structural configuration endows DMAP with several key properties:
- High Nucleophilicity: The enhanced electron density on the nitrogen atom makes DMAP an exceptionally reactive nucleophile, facilitating its interaction with electrophilic species.
- Strong Base: The dimethylamino group acts as a strong electron-donating group, increasing the basicity of the pyridine ring. This property enables DMAP to abstract protons, promoting reaction pathways.
- Excellent Leaving Group: The dimethylamino group, when attached to an acyl group, becomes a good leaving group, facilitating the formation of new bonds.
These properties, in concert, contribute to DMAP’s remarkable catalytic activity in a wide range of organic transformations.
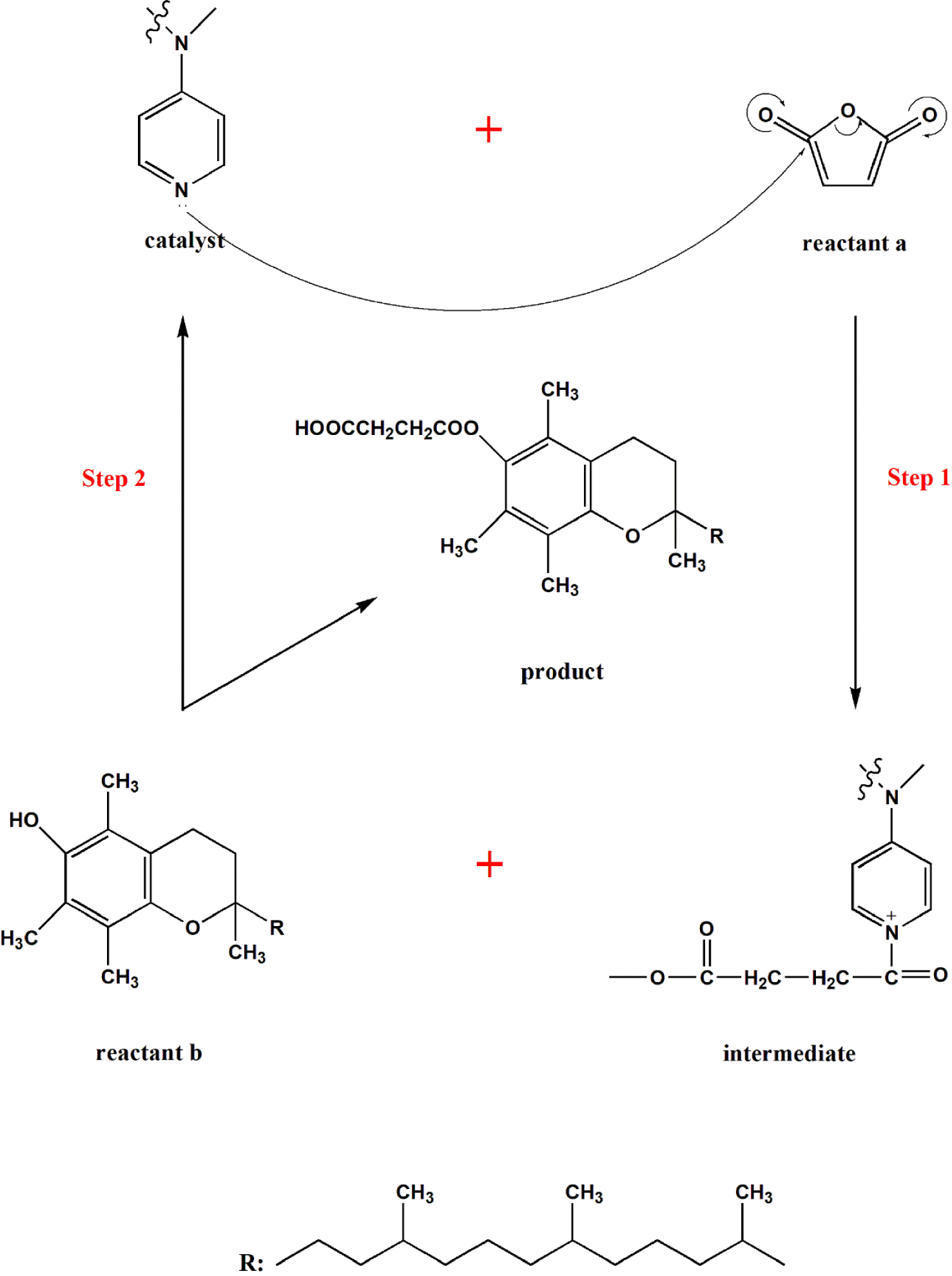
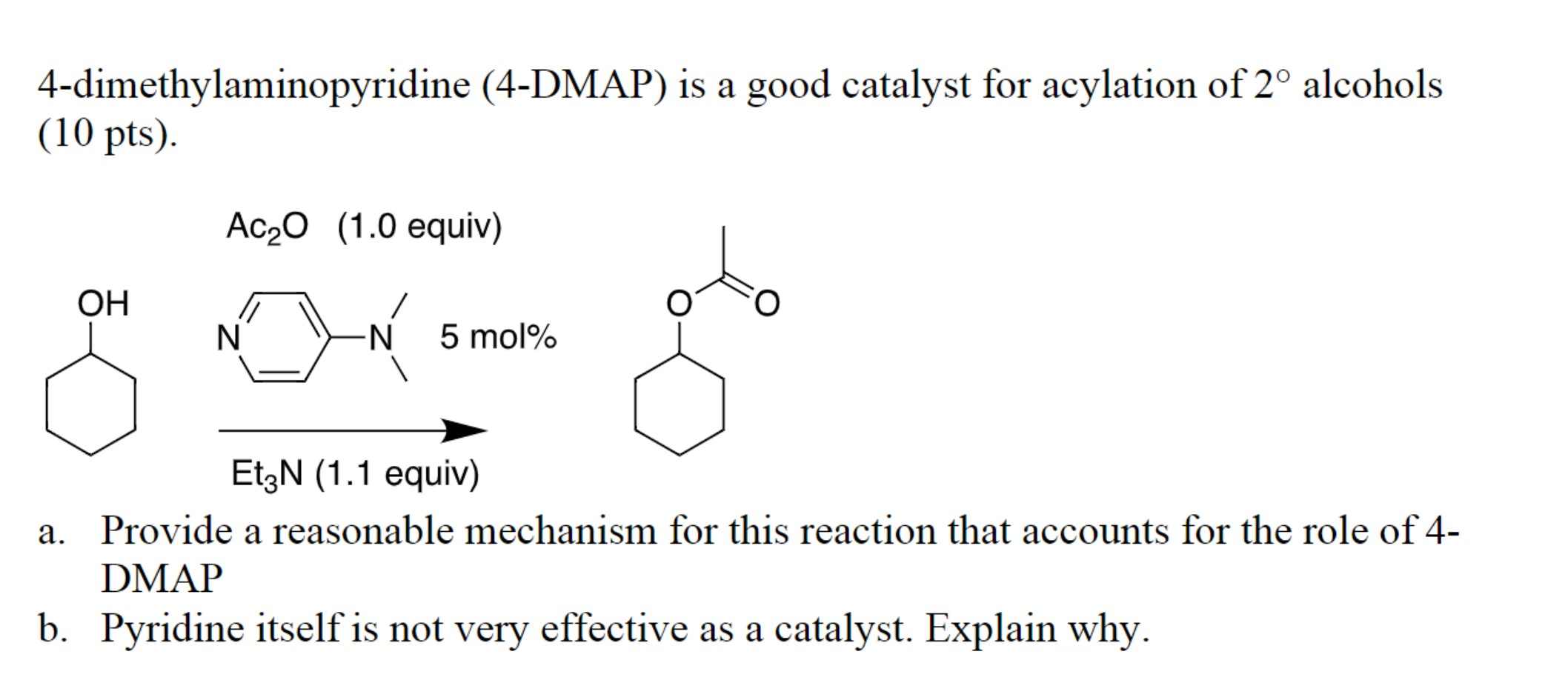
Deciphering the Mechanism of DMAP Catalysis
The catalytic prowess of DMAP is rooted in its ability to accelerate reactions by lowering the activation energy barrier. This is achieved through a multi-step mechanism involving nucleophilic attack, formation of an intermediate, and regeneration of the catalyst.
In a typical acyl transfer reaction, DMAP acts as a nucleophile, attacking the carbonyl group of an acyl halide or anhydride. This attack generates a tetrahedral intermediate, which is stabilized by the resonance effect. The resulting intermediate then undergoes a rapid elimination of the leaving group, regenerating DMAP and forming the desired acylated product.
The efficiency of this catalytic cycle is significantly enhanced by the following factors:
- Enhanced Nucleophilicity: The high nucleophilicity of DMAP ensures a rapid and efficient attack on the electrophilic carbonyl group.
- Stabilization of the Intermediate: The resonance effect stabilizes the tetrahedral intermediate, facilitating its formation and promoting the reaction rate.
- Good Leaving Group: The dimethylamino group, when attached to the acyl group, acts as a good leaving group, readily departing from the intermediate to regenerate the catalyst.
The combination of these factors makes DMAP a highly effective catalyst for a wide range of acyl transfer reactions, including esterification, amidation, and transesterification.
Applications of DMAP: A Catalyst for Diverse Transformations
DMAP’s versatility extends far beyond simple acyl transfer reactions. Its catalytic properties have found applications in numerous organic transformations, including:
- Acyl Transfer Reactions: DMAP is a highly efficient catalyst for esterification, amidation, and transesterification reactions. It accelerates the formation of esters, amides, and transesterified products by facilitating the transfer of acyl groups.
- Silylation Reactions: DMAP promotes the formation of silyl ethers by activating the hydroxyl group of alcohols, making them more susceptible to attack by silylating agents.
- Ring-Opening Reactions: DMAP catalyzes the ring-opening of cyclic carbonates and lactones, leading to the formation of valuable linear products.
- Nucleophilic Aromatic Substitution: DMAP can facilitate nucleophilic aromatic substitution reactions by activating the aromatic ring towards nucleophilic attack.
- C-H Activation: Recent studies have demonstrated DMAP’s ability to catalyze C-H activation reactions, opening up new avenues for functionalization of inert carbon-hydrogen bonds.
Advantages of DMAP: A Catalyst with Multiple Benefits
DMAP offers several advantages over traditional catalysts, making it a preferred choice for organic synthesis:
- High Efficiency: DMAP effectively accelerates reactions, leading to faster reaction times and improved yields.
- Mild Reaction Conditions: DMAP often facilitates reactions under milder conditions, reducing the need for harsh temperatures or strong acids/bases.
- Versatility: DMAP catalyzes a wide range of reactions, making it a versatile tool for diverse synthetic strategies.
- Selectivity: DMAP can exhibit high selectivity, promoting the formation of specific products while minimizing side reactions.
- Ease of Handling: DMAP is readily available, easy to handle, and can be used in both stoichiometric and catalytic amounts.
FAQs about DMAP: Addressing Common Questions
Q: What are the potential drawbacks of using DMAP as a catalyst?
A: While DMAP offers numerous advantages, it also has some drawbacks:
- Hygroscopicity: DMAP is hygroscopic, meaning it readily absorbs moisture from the air. This can affect its reactivity and stability, necessitating careful handling and storage.
- Sensitivity to Air and Moisture: DMAP can be sensitive to air and moisture, leading to degradation and reduced catalytic activity.
- Toxicity: DMAP is considered toxic and should be handled with appropriate safety precautions.
Q: How can I optimize the use of DMAP in my reaction?
A: Optimization of DMAP-catalyzed reactions involves factors like:
- Stoichiometry: The amount of DMAP used can significantly impact the reaction rate and yield.
- Solvent: The choice of solvent can affect the solubility of reactants and intermediates, influencing reaction kinetics.
- Temperature: Optimizing the reaction temperature can maximize reaction rate while minimizing side reactions.
Q: Are there any alternatives to DMAP as a catalyst?
A: Several alternatives to DMAP exist, including:
- 4-Pyrrolidinopyridine (PPY): PPY exhibits similar catalytic activity to DMAP and is often used as an alternative.
- 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU): DBU is a stronger base than DMAP and can be effective for certain reactions.
- N-Heterocyclic Carbenes (NHCs): NHCs are a class of highly efficient catalysts that have emerged as alternatives to DMAP.
Q: What are the future prospects of DMAP as a catalyst?
A: The field of organic catalysis is constantly evolving, and DMAP continues to play a crucial role in this advancement. Researchers are exploring new applications and modifications of DMAP to enhance its efficiency, selectivity, and sustainability.
Tips for Using DMAP as a Catalyst: Practical Guidance
- Storage: Store DMAP in a tightly sealed container under inert atmosphere to prevent degradation due to moisture and air exposure.
- Purification: Purify DMAP before use by recrystallization from hexane or toluene to ensure optimal catalytic activity.
- Solvent Selection: Choose a suitable solvent that dissolves both the reactants and DMAP while minimizing its degradation.
- Temperature Control: Monitor the reaction temperature to ensure optimal conditions and minimize side reactions.
- Stoichiometry Optimization: Determine the optimal amount of DMAP required for the desired reaction using experimental optimization.
- Safety Precautions: Handle DMAP with appropriate safety precautions, wearing gloves and working in a well-ventilated area.
Conclusion: The Enduring Importance of DMAP in Organic Synthesis
DMAP has emerged as a cornerstone of modern organic synthesis, offering a powerful and versatile tool for accelerating reactions and enhancing synthetic efficiency. Its unique structural features, coupled with its remarkable catalytic properties, have made it an indispensable catalyst for a wide range of transformations. While new catalysts are continually being developed, DMAP remains a valuable and widely employed reagent in both academic and industrial settings. Its enduring significance lies in its ability to simplify synthetic pathways, improve product yields, and facilitate the development of new and innovative organic molecules. DMAP’s continued evolution and application in organic synthesis promises to shape the future of this field, driving the discovery of novel molecules and materials with diverse applications.




![[PDF] 4-Dimethylaminopyridine as a catalyst in heroin synthesis. Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/bd2da3cfe7d11eafd93ccc26ca55c536e20d40f0/3-Figure2-1.png)

Closure
Thus, we hope this article has provided valuable insights into 4-Dimethylaminopyridine: A Versatile Catalyst in Organic Synthesis. We hope you find this article informative and beneficial. See you in our next article!